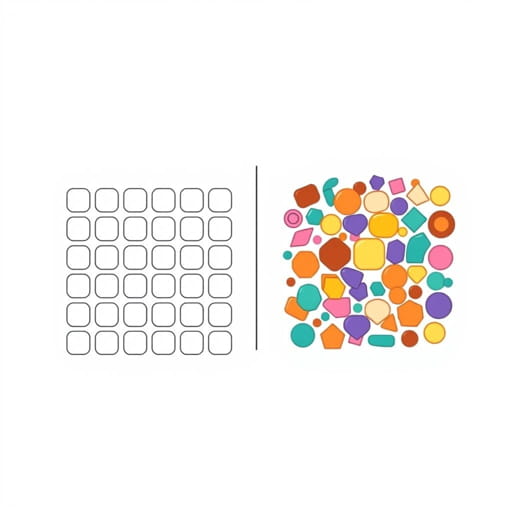
In the study of materials, mixtures, and various scientific systems, understanding the concepts of homogeneity and heterogeneity is essential. These terms describe the uniformity or diversity within a substance, mixture, or population and play a crucial role in chemistry, biology, physics, and even social sciences. Differentiating between homogeneous and heterogeneous systems helps in analyzing reactions, predicting behaviors, and applying the appropriate methods for separation, study, or utilization.
Defining Homogeneity
Homogeneity refers to a state in which a substance or mixture has a uniform composition and properties throughout. In a homogeneous system, every part of the material is identical in terms of chemical composition, physical properties, and structure. This uniformity ensures that any sample taken from the system will exhibit the same characteristics as any other sample from the same system. Homogeneous systems are often termed as solutions in chemistry, where one substance is completely dissolved in another, creating a consistent mixture at the molecular or atomic level.
Key Features of Homogeneous Systems
- Uniform CompositionThe material or mixture is consistent throughout, without visible boundaries or phases.
- Single PhaseHomogeneous systems typically exist in a single phase, whether solid, liquid, or gas.
- Indistinguishable ComponentsThe individual components are not visually separable.
- Predictable PropertiesPhysical and chemical properties are uniform and predictable across the entire system.
Examples of Homogeneous Systems
- Salt dissolved in water forming a saline solution.
- Air as a mixture of gases including oxygen, nitrogen, and carbon dioxide.
- Alloy metals like brass, a mixture of copper and zinc.
- Vinegar, a mixture of acetic acid and water.
Defining Heterogeneity
Heterogeneity refers to a state where a substance or mixture is composed of distinctly different components that are not uniformly distributed. In heterogeneous systems, the properties and composition vary from one region to another. Such systems often display multiple phases, and the individual components can frequently be observed or separated through mechanical means. Heterogeneous mixtures are common in everyday life and scientific applications, providing examples of complex systems that require careful analysis to understand their behavior fully.
Key Features of Heterogeneous Systems
- Non-Uniform CompositionDifferent regions of the material have distinct compositions and properties.
- Multiple PhasesHeterogeneous systems often contain more than one phase, such as solid-liquid, liquid-liquid, or solid-gas combinations.
- Visible ComponentsThe individual constituents can often be seen or separated physically.
- Variable PropertiesPhysical and chemical properties can differ depending on the sampled region of the system.
Examples of Heterogeneous Systems
- Sand mixed with water, where sand ptopics are distinct from water.
- Oil and water, which form separate layers due to immiscibility.
- Granite, a rock with visibly different minerals like quartz, feldspar, and mica.
- Salad or mixed vegetables, where individual ingredients retain their distinct properties.
Comparing Homogeneity and Heterogeneity
While both terms describe the composition of materials or systems, the key differences lie in uniformity, phase distribution, and visibility of components. Homogeneous systems exhibit consistent characteristics, often at the molecular or atomic level, and exist in a single phase. Heterogeneous systems, on the other hand, are characterized by variation in composition and properties, multiple phases, and often visibly distinct components.
Structural and Physical Differences
- PhaseHomogeneous systems are single-phase, whereas heterogeneous systems are multi-phase.
- Component VisibilityComponents in homogeneous mixtures cannot be seen with the naked eye, while heterogeneous mixtures often display visible constituents.
- Sample UniformityAny sample from a homogeneous system is representative of the whole, unlike in heterogeneous systems where sample properties may vary.
- Separation MethodsHomogeneous mixtures often require techniques like distillation or chromatography for separation, whereas heterogeneous mixtures can be separated physically, such as by filtration or decantation.
Chemical and Biological Implications
In chemistry, understanding homogeneity is crucial for reactions, as reactants in a uniform medium interact predictably. Homogeneous catalysts, solutions, and reagents ensure consistent reaction rates and outcomes. Heterogeneous systems, such as catalysts with distinct surfaces or mixtures with separate phases, influence reaction mechanisms differently, often providing sites for localized reactions or facilitating separation of products.
In biology, homogeneity and heterogeneity also play roles in cellular environments, ecosystems, and population studies. Homogeneous cell cultures provide uniform conditions for experiments, ensuring reproducibility. In contrast, heterogeneous populations or ecosystems display diversity in traits, behaviors, or species distribution, affecting interactions, adaptation, and evolutionary processes.
Applications in Industry and Research
Understanding the difference between homogeneous and heterogeneous systems is vital across industries. In pharmaceuticals, homogeneous solutions ensure precise dosing, while heterogeneous formulations like suspensions allow controlled release of active ingredients. In materials science, alloys represent homogeneous mixtures, whereas composite materials exemplify heterogeneity, combining distinct phases to achieve desired mechanical properties. Environmental sciences study heterogeneous soil compositions, water-sediment mixtures, and air pollutants to manage natural resources effectively.
Industrial Examples
- Homogeneous Sugar solution in beverages, where consistent sweetness is desired.
- Heterogeneous Concrete, composed of cement, sand, and gravel with distinct phases contributing to strength.
- Homogeneous Engine oils with uniform additives for consistent lubrication.
- Heterogeneous Emulsions in food processing, such as mayonnaise, where oil and water phases coexist.
Importance in Scientific Analysis
Accurate identification of homogeneity or heterogeneity is critical for designing experiments, analyzing data, and applying appropriate measurement techniques. Homogeneous systems allow for reproducible measurements and simplified modeling. Heterogeneous systems require careful sampling, consideration of phase distribution, and understanding of interactions between different components to ensure valid conclusions.
Analytical Techniques
- Homogeneous SystemsTechniques like spectroscopy, chromatography, and titration are effective due to uniform composition.
- Heterogeneous SystemsTechniques such as microscopy, sieving, centrifugation, and filtration help analyze distinct components.
In summary, homogeneity and heterogeneity describe the uniformity and diversity of systems in terms of composition, structure, and properties. Homogeneous systems are consistent, single-phase, and exhibit uniform properties throughout, making them ideal for predictable reactions and analytical methods. Heterogeneous systems consist of multiple phases, display variable properties, and often contain visible or separable components, requiring specialized approaches for study and application. Understanding these differences is essential in chemistry, biology, physics, materials science, and various industrial applications. Recognizing whether a system is homogeneous or heterogeneous informs experimental design, influences reaction outcomes, and guides practical applications, highlighting the fundamental importance of these concepts in science and everyday life.